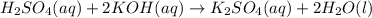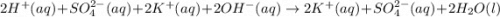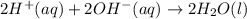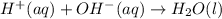Chemistry, 12.07.2019 02:30 kimberlyvazquez1121
Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of h2so4 and koh are mixed. give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of h2so4 and koh are mixed. 2 k+(aq) + so42-(aq) → k2so4(s) h+(aq) + oh-(aq) → h2o(l) h22+(aq) + oh-(aq) → h2(oh)2(l) h+(aq) + oh-(aq) + 2 k+(aq) + so42-(aq) → h2o(l) + k2so4(s) no reaction occurs.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2
You know the right answer?
Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of h2so4 an...
Questions







Physics, 19.02.2021 18:40


Business, 19.02.2021 18:40


Mathematics, 19.02.2021 18:40

Chemistry, 19.02.2021 18:40

Physics, 19.02.2021 18:40







Mathematics, 19.02.2021 18:40

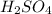 and
and 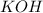 is:
is: