
Chemists often use molarity m, in moles/liter, to measure the concentration of solutions. molarity is a common unit of concentration because the volume of a liquid is very easy to measure. however, the drawback of using molarity is that volume is a temperature-dependent quantity. as temperature changes, density changes, which affects volume. volume markings for most laboratory glassware are calibrated for room temperature, about 20∘c. fortunately, there are several other ways of expressing concentration that do not involve volume and are therefore temperature independent. a 2.800×10−2 m solution of nacl in water is at 20.0∘c. the sample was created by dissolving a sample of nacl in water and then bringing the volume up to 1.000 l. it was determined that the volume of water needed to do this was 999.2 ml . the density of water at 20.0∘c is 0.9982 g/ml.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
Chemists often use molarity m, in moles/liter, to measure the concentration of solutions. molarity i...
Questions

Mathematics, 26.07.2020 01:01


Mathematics, 26.07.2020 01:01

Mathematics, 26.07.2020 01:01


Mathematics, 26.07.2020 01:01

Mathematics, 26.07.2020 01:01

English, 26.07.2020 01:01

Mathematics, 26.07.2020 01:01



Mathematics, 26.07.2020 01:01

Mathematics, 26.07.2020 01:01

Mathematics, 26.07.2020 01:01



Spanish, 26.07.2020 01:01

Spanish, 26.07.2020 01:01

Spanish, 26.07.2020 01:01

Mathematics, 26.07.2020 01:01

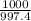 x 2.800×10⁻² moles of NaCl.
x 2.800×10⁻² moles of NaCl.

