
Chemistry, 12.07.2019 02:30 jdkrisdaimcc11
Aluminum hydroxide reacts with sulfuric acid as follows: 2 al(oh)3(s) + 3 h2so4(aq) → al2(so4)3(aq) + 6 h2o(l). which reagent is the limiting reactant when 0.410 mol al(oh)3 and 0.410 mol h2so4 are allowed to react? h2so4 al2(so4)3 h2o al(oh)3 correct: your answer is correct. how many moles of al2(so4)3 can form under these conditions? 0.137 correct: your answer is correct. mol how many moles of the excess reactant remain after the completion of the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
Aluminum hydroxide reacts with sulfuric acid as follows: 2 al(oh)3(s) + 3 h2so4(aq) → al2(so4)3(aq)...
Questions

Business, 02.07.2019 10:30


Mathematics, 02.07.2019 10:30

History, 02.07.2019 10:30

Mathematics, 02.07.2019 10:30


Mathematics, 02.07.2019 10:30




Business, 02.07.2019 10:30

Spanish, 02.07.2019 10:30

History, 02.07.2019 10:30








 gives 1 mole of
gives 1 mole of  thus, 1 mole of
thus, 1 mole of 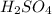 gives 1 mole of
gives 1 mole of 


