
Chemistry, 12.07.2019 05:30 KitKatKrunchy
Amixture of fe2o3 and feo was found to contain 72.00% fe by mass. what is the mass of fe2o3 in 0.0493 g of this mixture?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
You know the right answer?
Amixture of fe2o3 and feo was found to contain 72.00% fe by mass. what is the mass of fe2o3 in 0.049...
Questions

Business, 11.11.2020 20:10


Mathematics, 11.11.2020 20:10

History, 11.11.2020 20:10


Spanish, 11.11.2020 20:10

Mathematics, 11.11.2020 20:10

Mathematics, 11.11.2020 20:10

Spanish, 11.11.2020 20:10





Mathematics, 11.11.2020 20:10


German, 11.11.2020 20:10


Physics, 11.11.2020 20:10

Mathematics, 11.11.2020 20:10

Mathematics, 11.11.2020 20:10

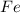 found in the mixture of
found in the mixture of 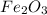 and
and 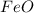 is 72.00 %. (Given)
is 72.00 %. (Given)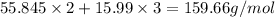
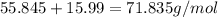
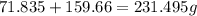
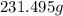 of mixture contains
of mixture contains 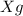 of
of 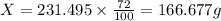
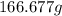 of
of 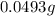 of
of 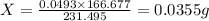
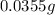 .
.

