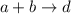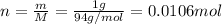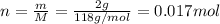Chemistry, 12.07.2019 05:30 risolatziyovudd
If 1.00 g of reactant a is mixed with 2.00 g of reactant b, what is the theoretical yield of product d, in grams (g)? the molar mass of a = 94.0 g/mol, b = 118 g/mol , and d = 125 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

You know the right answer?
If 1.00 g of reactant a is mixed with 2.00 g of reactant b, what is the theoretical yield of product...
Questions







History, 06.04.2020 03:25

Mathematics, 06.04.2020 03:25

History, 06.04.2020 03:25

Mathematics, 06.04.2020 03:25

Health, 06.04.2020 03:25




English, 06.04.2020 03:25




English, 06.04.2020 03:25

Mathematics, 06.04.2020 03:25