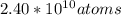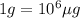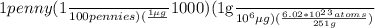Chemistry, 12.07.2019 06:00 danielmartinez91
The element californium (cf) sells for $1000 per µg. assuming 6.02 x 1023 atoms of cf have a mass of 251 grams, how many atoms of cf could you buy for 1 u. s. penny?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
The element californium (cf) sells for $1000 per µg. assuming 6.02 x 1023 atoms of cf have a mass of...
Questions

Mathematics, 24.09.2019 15:30




Biology, 24.09.2019 15:30

Mathematics, 24.09.2019 15:30


Mathematics, 24.09.2019 15:30

Chemistry, 24.09.2019 15:30




History, 24.09.2019 15:30

Mathematics, 24.09.2019 15:30