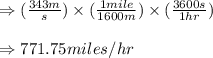Chemistry, 12.07.2019 06:30 cheervolley
The speed of the sound in air at room temperature is 343 m/s. calculate this speed in miles per hour.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1
You know the right answer?
The speed of the sound in air at room temperature is 343 m/s. calculate this speed in miles per hour...
Questions



Chemistry, 18.06.2020 19:57



Mathematics, 18.06.2020 19:57

Mathematics, 18.06.2020 19:57

Mathematics, 18.06.2020 19:57

English, 18.06.2020 19:57

Mathematics, 18.06.2020 19:57

Mathematics, 18.06.2020 19:57



Biology, 18.06.2020 19:57