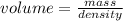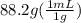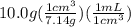Chemistry, 12.07.2019 08:30 nickboy52210
An empty flask has a mass of 123.4 g. when the flask is filled with water, the madd is 211.6g. if 10.0g of zinc (d=7.14 g/cm^3) are added to the flask filled with water (and the sides of the flask are dried from the displaced water) what is the new volume in the flask? (hint: you are going to have to use the density of water to answer this question)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

You know the right answer?
An empty flask has a mass of 123.4 g. when the flask is filled with water, the madd is 211.6g. if 10...
Questions

Mathematics, 05.05.2020 14:58




Biology, 05.05.2020 14:58


Chemistry, 05.05.2020 14:58

Biology, 05.05.2020 14:58



English, 05.05.2020 14:58


History, 05.05.2020 14:58

Mathematics, 05.05.2020 14:58

Mathematics, 05.05.2020 14:58

Mathematics, 05.05.2020 14:58



Mathematics, 05.05.2020 14:58

Mathematics, 05.05.2020 14:58

 .
.