
Chemistry, 12.07.2019 09:30 qudoniselmore0
Complete combustion of 1.5 g of fructose a sugar that contains carbon, hydrogen, and oxygen yields 2.2 g of carbon dioxide and .9 g of water. determine the empirical formula of fructose

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1


Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Complete combustion of 1.5 g of fructose a sugar that contains carbon, hydrogen, and oxygen yields 2...
Questions

History, 20.07.2019 21:00

Mathematics, 20.07.2019 21:00






History, 20.07.2019 21:00





Mathematics, 20.07.2019 21:00


English, 20.07.2019 21:00


Spanish, 20.07.2019 21:00



History, 20.07.2019 21:00

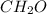 .
.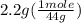 = 0.05 mole
= 0.05 mole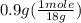 = 0.05 mole
= 0.05 mole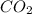 has one C means the mole ratio of
has one C means the mole ratio of 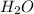 has two H means the mole ratio of
has two H means the mole ratio of 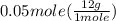 = 0.6 g
= 0.6 g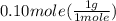 = 0.1g
= 0.1g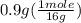 = 0.056 moles
= 0.056 moles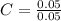 = 1
= 1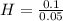 = 2
= 2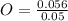 = 1
= 1

