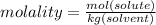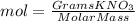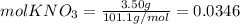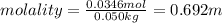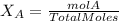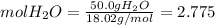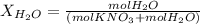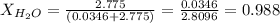Chemistry, 12.07.2019 12:00 Pizzapegasus1
Calculate the molality and mole fraction of water, respectively, of a solution that is made by dissolving 3.50 g of potassium nitrate in 50.0 g of water. the final volume of the solution is 56.0 ml.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
Calculate the molality and mole fraction of water, respectively, of a solution that is made by disso...
Questions



Mathematics, 29.06.2019 14:30

History, 29.06.2019 14:30


English, 29.06.2019 14:30


Arts, 29.06.2019 14:30

English, 29.06.2019 14:30



Mathematics, 29.06.2019 14:30


History, 29.06.2019 14:30


History, 29.06.2019 14:30