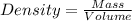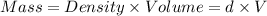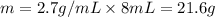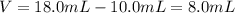Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3
You know the right answer?
Asample of aluminum is placed in a 25-ml graduated cylinder containing 10.0 ml of water. the level o...
Questions


Computers and Technology, 29.01.2020 07:05


English, 29.01.2020 07:05

Health, 29.01.2020 07:05

English, 29.01.2020 07:05

Biology, 29.01.2020 07:05


Mathematics, 29.01.2020 07:05

Mathematics, 29.01.2020 07:05




Mathematics, 29.01.2020 07:05


Biology, 29.01.2020 07:05

Social Studies, 29.01.2020 07:05

Mathematics, 29.01.2020 07:05

Biology, 29.01.2020 07:05