
Chemistry, 12.07.2019 14:30 pattydixon6
What is the empirical formula of an unknown compound that contains 60.3% of magnesium and 39.7% of oxygen?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2


Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2

You know the right answer?
What is the empirical formula of an unknown compound that contains 60.3% of magnesium and 39.7% of o...
Questions

Mathematics, 16.08.2021 16:30

Mathematics, 16.08.2021 16:30


Chemistry, 16.08.2021 16:30

Mathematics, 16.08.2021 16:30

Mathematics, 16.08.2021 16:30

Social Studies, 16.08.2021 16:30



Social Studies, 16.08.2021 16:30

Chemistry, 16.08.2021 16:30

Social Studies, 16.08.2021 16:30



Mathematics, 16.08.2021 16:30

Mathematics, 16.08.2021 16:30

Biology, 16.08.2021 16:30

Social Studies, 16.08.2021 16:30

History, 16.08.2021 16:40

Chemistry, 16.08.2021 16:40

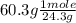 = 2.48 mole
= 2.48 mole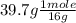 = 2.48 moles
= 2.48 moles

