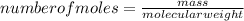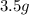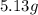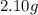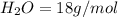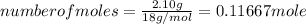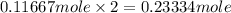Chemistry, 12.07.2019 15:00 jsully5159
An unknown compound contains only carbon, hydrogen, and oxygen (cxhyoz). combustion of 3.50 g of this compound produced 5.13 g of carbon dioxide and 2.10 g of water. how many moles of hydrogen, h, were in the original sample?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3
You know the right answer?
An unknown compound contains only carbon, hydrogen, and oxygen (cxhyoz). combustion of 3.50 g of thi...
Questions


Social Studies, 24.11.2020 22:10

Chemistry, 24.11.2020 22:10


English, 24.11.2020 22:10


English, 24.11.2020 22:10

Physics, 24.11.2020 22:10

Chemistry, 24.11.2020 22:10



Mathematics, 24.11.2020 22:10




Arts, 24.11.2020 22:10




Geography, 24.11.2020 22:10