Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2
You know the right answer?
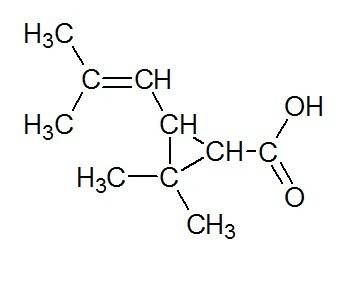
Chrysanthemic acid is isolated from chrysanthemum flowers. the ir spectrum of chrysanthemic acid exh...
Questions

Mathematics, 28.08.2020 01:01

Mathematics, 28.08.2020 01:01









Mathematics, 28.08.2020 01:01

Chemistry, 28.08.2020 01:01

Mathematics, 28.08.2020 01:01







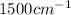 are:
are: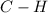 bond (
bond (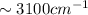 ).
).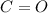 bond (
bond (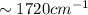 ).
).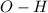 bond of carboxylic acid (
bond of carboxylic acid (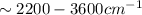 )
)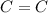 bond (
bond (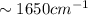 )
)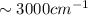 ).
).