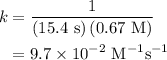Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
The half-life for the second-order decomposition of hi is 15.4 s when the initial concentration of h...
Questions



Mathematics, 24.10.2020 01:00




History, 24.10.2020 01:00


Biology, 24.10.2020 01:00


Biology, 24.10.2020 01:00

Social Studies, 24.10.2020 01:00



Biology, 24.10.2020 01:00

Mathematics, 24.10.2020 01:00


Mathematics, 24.10.2020 01:00


Biology, 24.10.2020 01:00

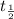 =
=![\frac{1}{k[A_o]}](/tpl/images/0081/7570/1cf43.png) (1)
(1)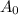 = initial concentration
= initial concentration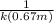
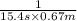
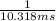
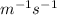
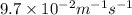
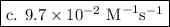 .
.
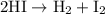
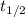 .
.
![{t_{{\text{1/2}}}} = \dfrac{1}{{k\left[ {{{\text{A}}_{\text{o}}}} \right]}}](/tpl/images/0081/7570/8ca83.png) …… (1)
…… (1)
![\left[ {{{\text{A}}_{\text{o}}}} \right]](/tpl/images/0081/7570/38db1.png) is the initial concentration of reactant.
is the initial concentration of reactant.
![k = \dfrac{1}{{{t_{{\text{1/2}}}}\left[ {{{\text{A}}_{\text{o}}}} \right]}}](/tpl/images/0081/7570/d5e88.png) …… (2)
…… (2)