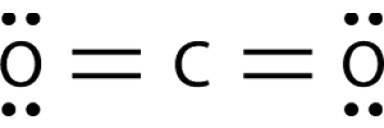Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
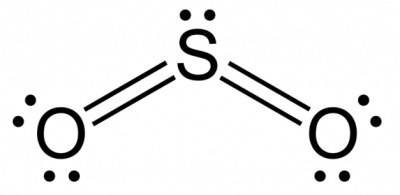
Bonds between carbon and oxygen (c=o) are more polar than bonds between sulfur and oxygen (s=o). nev...
Questions

Mathematics, 27.09.2021 19:50

Mathematics, 27.09.2021 19:50


Computers and Technology, 27.09.2021 19:50




English, 27.09.2021 19:50


Computers and Technology, 27.09.2021 19:50

Computers and Technology, 27.09.2021 19:50

Social Studies, 27.09.2021 19:50

Mathematics, 27.09.2021 19:50


Mathematics, 27.09.2021 19:50



History, 27.09.2021 19:50


Mathematics, 27.09.2021 19:50