

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
You know the right answer?
Cadmium metal reacts vigorously with yellow crystals of sulfur to produce a yellow powder of cadmium...
Questions


Mathematics, 05.05.2021 02:20

Mathematics, 05.05.2021 02:20

Mathematics, 05.05.2021 02:20



Social Studies, 05.05.2021 02:20


Geography, 05.05.2021 02:20

History, 05.05.2021 02:20



Mathematics, 05.05.2021 02:20




Mathematics, 05.05.2021 02:20

Mathematics, 05.05.2021 02:20

Mathematics, 05.05.2021 02:20

Mathematics, 05.05.2021 02:20

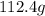 of cadmium with
of cadmium with 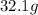 of sulfur =
of sulfur = 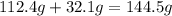
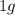 of cadmium forms
of cadmium forms 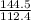 cadmium sulfide.
cadmium sulfide.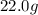 of cadmium will give
of cadmium will give 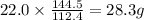 of cadmium sulfide.
of cadmium sulfide.

