

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2


Chemistry, 23.06.2019 12:50
Acertain reaction has a activation energy of 54.0 kj/mol. as the temperature is increased from 22c to a higher temperature, the rate constant increases by a factor of 7.00. calculate the higher temperature. c (report only numerical answer)
Answers: 3
You know the right answer?
What mass of h2 is needed to react with 8.75 g of o2 according to the following equation: o2(g) + h...
Questions


Geography, 16.12.2020 14:00

Mathematics, 16.12.2020 14:00

Arts, 16.12.2020 14:00


Mathematics, 16.12.2020 14:00

History, 16.12.2020 14:00

History, 16.12.2020 14:00

Arts, 16.12.2020 14:00

Mathematics, 16.12.2020 14:00


English, 16.12.2020 14:00

Arts, 16.12.2020 14:00

Mathematics, 16.12.2020 14:00

Mathematics, 16.12.2020 14:00

Mathematics, 16.12.2020 14:00

English, 16.12.2020 14:00

Mathematics, 16.12.2020 14:00

Geography, 16.12.2020 14:00

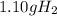 is the right answer.
is the right answer.



