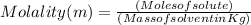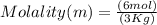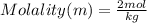Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
What is the molality of a solution made by dissolving 6 moles of naoh in 3 kg of water?...
Questions

History, 18.03.2021 01:40



Mathematics, 18.03.2021 01:40


History, 18.03.2021 01:40

History, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40

Chemistry, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40







English, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40