
Chemistry, 12.07.2019 21:00 kotetravels10
Asample of an unknown substance contains 38.43 g lead, 17.83 g carbon, and 3.74 g hydrogen. what is the empirical formula? a: pbc4h10 b: pb2c8h15 c: pbc8h20 d: pb2c4h15

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1


Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Asample of an unknown substance contains 38.43 g lead, 17.83 g carbon, and 3.74 g hydrogen. what is...
Questions

English, 15.05.2021 02:30

Biology, 15.05.2021 02:30




Mathematics, 15.05.2021 02:30

Health, 15.05.2021 02:30







Mathematics, 15.05.2021 02:30

Mathematics, 15.05.2021 02:30

Mathematics, 15.05.2021 02:30

Mathematics, 15.05.2021 02:30

Chemistry, 15.05.2021 02:30


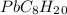 .
.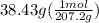
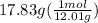
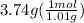
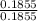 = 1
= 1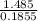 = 8
= 8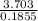 = 20
= 20

