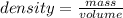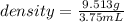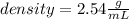Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
You drop a marble ball into a glass filled with water, which results ib 3.75 ml of water being displ...
Questions

History, 03.12.2020 02:50

Mathematics, 03.12.2020 02:50

Mathematics, 03.12.2020 02:50


Mathematics, 03.12.2020 02:50

Chemistry, 03.12.2020 02:50


History, 03.12.2020 02:50


History, 03.12.2020 02:50



Mathematics, 03.12.2020 02:50

Mathematics, 03.12.2020 02:50


Mathematics, 03.12.2020 02:50


Mathematics, 03.12.2020 02:50


Mathematics, 03.12.2020 02:50