

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1
You know the right answer?
The equation below shows hydrogen reacting with oxygen to produce water. 2h2 + o2 2h2o if 16 mol of...
Questions

Mathematics, 21.10.2019 23:30

Mathematics, 21.10.2019 23:30

Mathematics, 21.10.2019 23:30

Advanced Placement (AP), 21.10.2019 23:30


Mathematics, 21.10.2019 23:30

Physics, 21.10.2019 23:30

Mathematics, 21.10.2019 23:30

Mathematics, 21.10.2019 23:30

History, 21.10.2019 23:30



Mathematics, 21.10.2019 23:30


Computers and Technology, 21.10.2019 23:30

Health, 21.10.2019 23:30


Mathematics, 21.10.2019 23:30


Mathematics, 21.10.2019 23:30

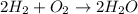
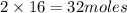 of water
of water

