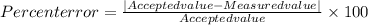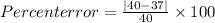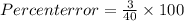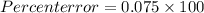Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

You know the right answer?
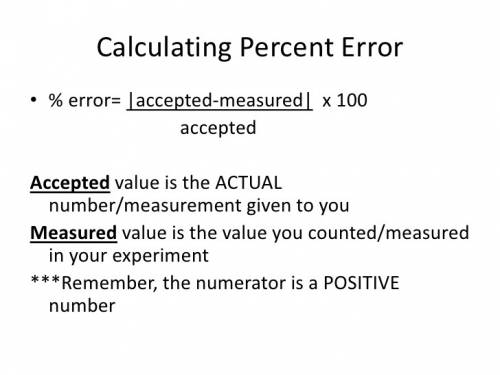
Ateacher calculates the molar mass of sodium hydroxide as 37 g/mol. the true molar mass of sodium hy...
Questions


Mathematics, 08.03.2021 22:10







Physics, 08.03.2021 22:10

Mathematics, 08.03.2021 22:10



Mathematics, 08.03.2021 22:10

Mathematics, 08.03.2021 22:10

Mathematics, 08.03.2021 22:10



History, 08.03.2021 22:10