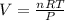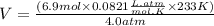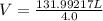Chemistry, 13.07.2019 00:30 Isaiahtate053
What is the volume of 6.9 mol oxygen (o2) gas at 233 k and a pressure of 4.0 atm? (the universal gas constant is 0.0821 l•atm/mol•k.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
What is the volume of 6.9 mol oxygen (o2) gas at 233 k and a pressure of 4.0 atm? (the universal ga...
Questions



Arts, 20.06.2021 22:30

Mathematics, 20.06.2021 22:30


Mathematics, 20.06.2021 22:30




World Languages, 20.06.2021 22:30



Spanish, 20.06.2021 22:30

Mathematics, 20.06.2021 22:30

Computers and Technology, 20.06.2021 22:30