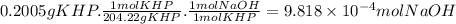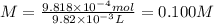Chemistry, 13.07.2019 00:30 tookie6208
In part 1 of lab 2 you will make and standardize a solution of naoh(aq). suppose in the lab you measure the solid naoh and dissolve it into 100.0 ml of water. you then measure 0.2005 g of khp (kc8h5o4, 204.22 g/mol) and place it in a clean, dry 100-ml beaker, and then dissolve the khp in about 25 ml of water and add a couple of drops of phenolphthalein indicator. you titrate this with your naoh(aq) solution and find that the titration requires 9.82 ml of naoh(aq).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
In part 1 of lab 2 you will make and standardize a solution of naoh(aq). suppose in the lab you meas...
Questions




Geography, 30.01.2020 11:46

Mathematics, 30.01.2020 11:46

Mathematics, 30.01.2020 11:46

Social Studies, 30.01.2020 11:46



Geography, 30.01.2020 11:46






Mathematics, 30.01.2020 11:46


Social Studies, 30.01.2020 11:46