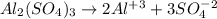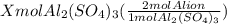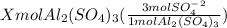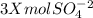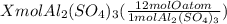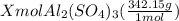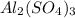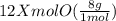You have x moles of al2(so4)3. express each of the following quantities in terms of x. a) the number of moles of aluminum ions b) the number of moles of sulfate ions c) the number of moles of oxygen atoms d) the number of grams of al2(so4)3 e) the number of grams of oxygen

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

You know the right answer?
You have x moles of al2(so4)3. express each of the following quantities in terms of x. a) the numbe...
Questions

Engineering, 04.11.2020 05:40

Mathematics, 04.11.2020 05:40


Mathematics, 04.11.2020 05:40


Mathematics, 04.11.2020 05:40

Health, 04.11.2020 05:40


History, 04.11.2020 05:40

History, 04.11.2020 05:40

Mathematics, 04.11.2020 05:40

Biology, 04.11.2020 05:40

Mathematics, 04.11.2020 05:40

Mathematics, 04.11.2020 05:40

Mathematics, 04.11.2020 05:40


Mathematics, 04.11.2020 05:40