
Chemistry, 13.07.2019 07:00 jordynsmith02
List the following bond types in order of increasing strength: non-polar covalent bonds, ionic bonds, hydrogen bonds, polar covalent bonds. a. hydrogen bonds, ionic bonds, non-polar covalent bonds, polar covalent bonds. b. hydrogen bonds, non-polar covalent bonds, polar covalent bonds, ionic bonds. c. hydrogen bonds, ionic bonds, polar covalent bonds, non-polar covalent bonds. d. ionic bonds, polar covalent bonds, non-polar covalent bonds, hydrogen bondse. non-polar covalent bonds, polar covalent bonds, hydrogen bonds, ionic bonds.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

You know the right answer?
List the following bond types in order of increasing strength: non-polar covalent bonds, ionic bond...
Questions





Mathematics, 04.09.2020 22:01




English, 04.09.2020 22:01


Biology, 04.09.2020 22:01

Mathematics, 04.09.2020 22:01


Health, 04.09.2020 22:01


Mathematics, 04.09.2020 22:01

Mathematics, 04.09.2020 22:01



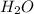 is a polar covalent compound. Partial opposite charges tend to develop on the atoms of a polar covalent compound.
is a polar covalent compound. Partial opposite charges tend to develop on the atoms of a polar covalent compound. 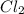 is a non-polar covalent molecule. No partial charges will be there on the atoms of a non-polar covalent molecule.
is a non-polar covalent molecule. No partial charges will be there on the atoms of a non-polar covalent molecule.

