
Chemistry, 13.07.2019 08:00 kennyjortiz
A0.4230 g sample of impure sodium nitrate (contains sodium nitrate plus inert ingredients) was heated, converting all the sodium nitrate to 0.2820 g of sodium nitrite and oxygen gas. determine the percent of sodium nitrate in the original sample.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
A0.4230 g sample of impure sodium nitrate (contains sodium nitrate plus inert ingredients) was heate...
Questions

Biology, 06.07.2019 02:00


Mathematics, 06.07.2019 02:00

Chemistry, 06.07.2019 02:00

History, 06.07.2019 02:00

Physics, 06.07.2019 02:00

Mathematics, 06.07.2019 02:00


English, 06.07.2019 02:00

Mathematics, 06.07.2019 02:00






Mathematics, 06.07.2019 02:00



Chemistry, 06.07.2019 02:00

Spanish, 06.07.2019 02:00


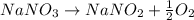
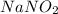 = 68.9953 g/mol
= 68.9953 g/mol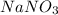 = 84.9947 g/mol
= 84.9947 g/mol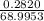 moles of
moles of 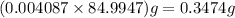
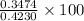 % =
% = 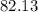 %
%

