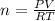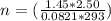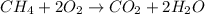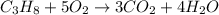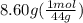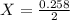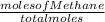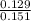A2.50-l flask contains a mixture of methane (ch4) and propane (c3h8) at a pressure of 1.45 atm and 20°c. when this gas mixture is then burned in excess oxygen, 8.60 g of carbon dioxide is formed. (the other product is water.) what is the mole fraction of methane in the original gas mixture?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
A2.50-l flask contains a mixture of methane (ch4) and propane (c3h8) at a pressure of 1.45 atm and 2...
Questions

Mathematics, 22.02.2021 20:10


Mathematics, 22.02.2021 20:10

Mathematics, 22.02.2021 20:10

Mathematics, 22.02.2021 20:10








History, 22.02.2021 20:10

Advanced Placement (AP), 22.02.2021 20:10

Chemistry, 22.02.2021 20:10

Mathematics, 22.02.2021 20:10

Biology, 22.02.2021 20:10


Advanced Placement (AP), 22.02.2021 20:10