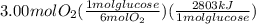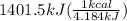Chemistry, 13.07.2019 08:00 zekrader18
The combustion of glucose (c6h12o6) with oxygen gas produces carbon dioxide and water. this process releases 2803 kj per mole of glucose. when 3.00 mol of oxygen react in this way with glucose, what is the energy release in kcal? (hint: write a balanced equation for the combustion process.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 06:30
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1

You know the right answer?
The combustion of glucose (c6h12o6) with oxygen gas produces carbon dioxide and water. this process...
Questions