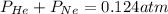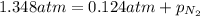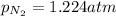Chemistry, 13.07.2019 09:30 shanicejordan
Amixture of he, ne, and n2 gases are a pressure of 1.348. if the pressures of he and ne are 0.124 atm, what is the partial pressure of n2 in the mixture ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
Amixture of he, ne, and n2 gases are a pressure of 1.348. if the pressures of he and ne are 0.124 at...
Questions


History, 26.07.2019 10:50


Social Studies, 26.07.2019 10:50


Mathematics, 26.07.2019 10:50

Social Studies, 26.07.2019 10:50

Mathematics, 26.07.2019 10:50



Mathematics, 26.07.2019 10:50


Health, 26.07.2019 10:50

Mathematics, 26.07.2019 10:50




Biology, 26.07.2019 10:50

Mathematics, 26.07.2019 10:50

Mathematics, 26.07.2019 10:50

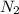 in the mixture is, 1.224 atm
in the mixture is, 1.224 atm
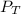 = total partial pressure of
= total partial pressure of 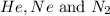 = 1.348 atm
= 1.348 atm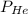 = partial pressure of helium
= partial pressure of helium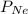 = partial pressure of neon
= partial pressure of neon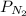 = partial pressure of nitrogen
= partial pressure of nitrogen