

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3
You know the right answer?
The gas with an initial volume of 24.0 l at a pressure of 565 mmhg is compressed until the volume is...
Questions

Computers and Technology, 08.12.2021 18:30



English, 08.12.2021 18:30

English, 08.12.2021 18:30



Engineering, 08.12.2021 18:30


Physics, 08.12.2021 18:30


Mathematics, 08.12.2021 18:30



Geography, 08.12.2021 18:30


Mathematics, 08.12.2021 18:30


English, 08.12.2021 18:30

Business, 08.12.2021 18:30

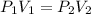
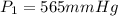
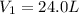
![V_2=16.0L[tex]P_2=?](/tpl/images/0084/3087/6fbf6.png)
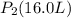
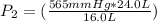
 = 848 mmHg
= 848 mmHg

