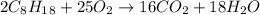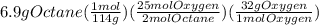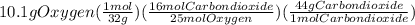Chemistry, 13.07.2019 09:30 cyaransteenberg
Problem page liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 6.9 g of octane is mixed with 10.1 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2
You know the right answer?
Problem page liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon di...
Questions




Mathematics, 25.08.2021 15:10



Mathematics, 25.08.2021 15:10


Mathematics, 25.08.2021 15:10


Chemistry, 25.08.2021 15:10

Mathematics, 25.08.2021 15:10



Computers and Technology, 25.08.2021 15:10

Social Studies, 25.08.2021 15:10

Physics, 25.08.2021 15:10

Business, 25.08.2021 15:10