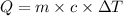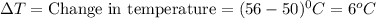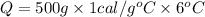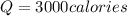Chemistry, 13.07.2019 11:30 lisapcarroll
How many calories are absorbed by a sample of water with a mass of 500 grams as it changes from 50°c to 56°c? the specific heat capacity of water is 1.00 cal/g°c. 83 calories 494 calories 506 calories 3,000 calories

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1
You know the right answer?
How many calories are absorbed by a sample of water with a mass of 500 grams as it changes from 50°c...
Questions


Chemistry, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40


Mathematics, 17.11.2020 17:40





History, 17.11.2020 17:40


Social Studies, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40

Physics, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40


Social Studies, 17.11.2020 17:40


Advanced Placement (AP), 17.11.2020 17:40

Social Studies, 17.11.2020 17:40