
Chemistry, 13.07.2019 12:30 codeyhatch142
One way to identify a substance is to compare the density of the substance with the density of a known substance. suppose a cube of a silver-colored metal with a volume of 64 cm3 has a mass of 672 g. the density of pure silver is known to be 10.5 g/cm3. based on this information, could the metal be pure silver? a. no. the density is greater than pure silver's. b. no. the density is less than pure silver's. c. yes. the density equals that of pure silver.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1
You know the right answer?
One way to identify a substance is to compare the density of the substance with the density of a kn...
Questions

Spanish, 07.04.2021 17:00

Mathematics, 07.04.2021 17:00


History, 07.04.2021 17:00

English, 07.04.2021 17:00

Mathematics, 07.04.2021 17:00

History, 07.04.2021 17:00

Mathematics, 07.04.2021 17:00


Mathematics, 07.04.2021 17:00

Biology, 07.04.2021 17:00


Biology, 07.04.2021 17:00

Mathematics, 07.04.2021 17:00


Mathematics, 07.04.2021 17:00

Spanish, 07.04.2021 17:00

Mathematics, 07.04.2021 17:00


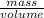 =
= 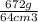 cm³
cm³


