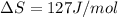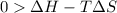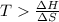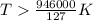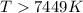Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1


Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
At what temperature is the following reaction feasible: hcl(g) + nh3(g) -> nh4cl(s)?
Questions




Mathematics, 25.06.2019 16:40





Mathematics, 25.06.2019 16:40

Mathematics, 25.06.2019 16:40

Mathematics, 25.06.2019 16:40





English, 25.06.2019 16:40




Health, 25.06.2019 16:40

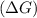 should be negative or
should be negative or 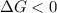 .
.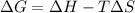
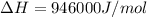 and
and