Chemistry, 13.07.2019 16:30 rerunkle96
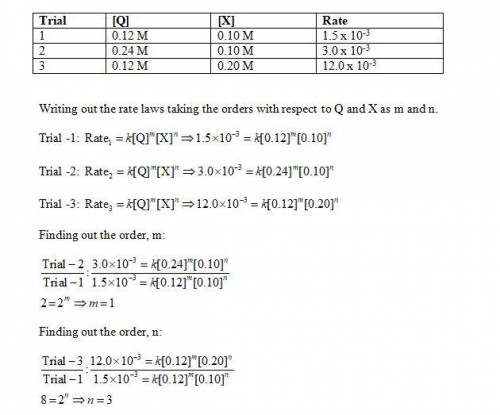
Determine the rate law, including the values of the orders and rate law constant, for the following reaction using the experimental data provided. q + x yields products trial [q] [x] rate 1 0.12 m 0.10 m 1.5 × 10-3 m/min 2 0.24 m 0.10 m 3.0 × 10-3 m/min 3 0.12 m 0.20 m 12.0 × 10-3 m/min

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

You know the right answer?
Determine the rate law, including the values of the orders and rate law constant, for the following...
Questions





Biology, 22.06.2021 15:30




Mathematics, 22.06.2021 15:30

Mathematics, 22.06.2021 15:30


Biology, 22.06.2021 15:30

History, 22.06.2021 15:30


Mathematics, 22.06.2021 15:30

Mathematics, 22.06.2021 15:30

Biology, 22.06.2021 15:30


Business, 22.06.2021 15:30