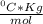Chemistry, 13.07.2019 18:30 sindy35111
Given that the freezing point depression constant for water is 1.86°c kg/mol, calculate the change in freezing point for a 0.907 m sugar solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

You know the right answer?
Given that the freezing point depression constant for water is 1.86°c kg/mol, calculate the change i...
Questions


Mathematics, 08.02.2021 16:30

Biology, 08.02.2021 16:30

Mathematics, 08.02.2021 16:30

Mathematics, 08.02.2021 16:30

English, 08.02.2021 16:30



Mathematics, 08.02.2021 16:30


History, 08.02.2021 16:30


English, 08.02.2021 16:30


Mathematics, 08.02.2021 16:30

Physics, 08.02.2021 16:30

Mathematics, 08.02.2021 16:30

Biology, 08.02.2021 16:30

Social Studies, 08.02.2021 16:30

Mathematics, 08.02.2021 16:30

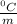
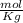 )
)