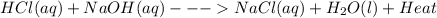Chemistry, 13.07.2019 18:30 preservations
When hcl(aq) and naoh(aq) are mixed in a beaker, the beaker feels warm to the touch. what is known about the enthalpy of this reaction? view available hint(s) when and are mixed in a beaker, the beaker feels warm to the touch. what is known about the enthalpy of this reaction? heat is absorbed from the surroundings. δh is positive. the reaction is exothermic. the reaction is endothermic?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1

Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1

Chemistry, 23.06.2019 07:30
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1
You know the right answer?
When hcl(aq) and naoh(aq) are mixed in a beaker, the beaker feels warm to the touch. what is known a...
Questions

History, 18.02.2021 01:00



Physics, 18.02.2021 01:00



Mathematics, 18.02.2021 01:00

History, 18.02.2021 01:00



Mathematics, 18.02.2021 01:00

Arts, 18.02.2021 01:00


Mathematics, 18.02.2021 01:00

Arts, 18.02.2021 01:00





Mathematics, 18.02.2021 01:00