
Chemistry, 13.07.2019 18:30 adyenamaie02
In part a, you found the amount of product (3.80 mol p2o5 ) formed from the given amount of phosphorus and excess oxygen. in part b, you found the amount of product (3.40 mol p2o5 ) formed from the given amount of oxygen and excess phosphorus. now, determine how many moles of p2o5 are produced from the given amounts of phosphorus and oxygen. express your answer to three significant figures and include the appropriate units. view available hint(s)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2
You know the right answer?
In part a, you found the amount of product (3.80 mol p2o5 ) formed from the given amount of phosphor...
Questions







Computers and Technology, 11.02.2020 03:49








Mathematics, 11.02.2020 03:49





Computers and Technology, 11.02.2020 03:50

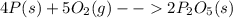
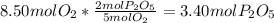
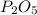 is formed from P (limiting reactant):
is formed from P (limiting reactant):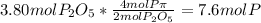
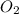 =
= 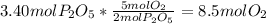
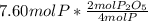
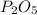 produced from P are
produced from P are 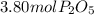
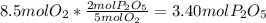
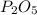 produced will be 3.40 mol
produced will be 3.40 mol

