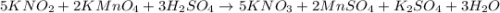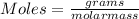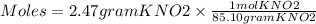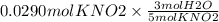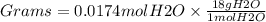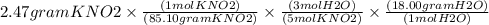Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Using the following equation 5kno2+2kmno4+3h2so4=5kno3+2mnso4+k2 so4+3h20. starting with 2.47 grams...
Questions



Biology, 03.09.2020 01:01


Mathematics, 03.09.2020 01:01

Mathematics, 03.09.2020 01:01

Mathematics, 03.09.2020 01:01




Mathematics, 03.09.2020 01:01


English, 03.09.2020 01:01

Computers and Technology, 03.09.2020 01:01

Social Studies, 03.09.2020 01:01

Mathematics, 03.09.2020 01:01

Mathematics, 03.09.2020 01:01


Chemistry, 03.09.2020 01:01