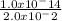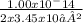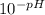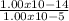Chemistry, 13.07.2019 22:30 rainbowmc6
Asap 1) determine the hydronium and hydroxide ion concentrations in a solution that is 2.0 × 10-2 m naoh 2) determine the ph of a solution that is 5.0 × 10-4 m hno3+ 3) determine the ph of a 3.45 × 10-2 m sr(oh)2 solution. 4) the ph of a solution is determined to be 7.0. what is the hydronium ion concentration of this solution? 5) the ph of an aqueous solution is measured as 5.00 calculate the [h3o+] and the [oh-]. i've got the answers for each but i don't know if i'm correct and if i used correctly significant figures. , !

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
Asap 1) determine the hydronium and hydroxide ion concentrations in a solution that is 2.0 × 10-2 m...
Questions


Mathematics, 20.10.2020 07:01


Mathematics, 20.10.2020 07:01

Mathematics, 20.10.2020 07:01

Mathematics, 20.10.2020 07:01

Mathematics, 20.10.2020 07:01

History, 20.10.2020 07:01


Mathematics, 20.10.2020 07:01

Chemistry, 20.10.2020 07:01


Business, 20.10.2020 07:01

Geography, 20.10.2020 07:01


Biology, 20.10.2020 07:01


History, 20.10.2020 07:01

Mathematics, 20.10.2020 07:01

Mathematics, 20.10.2020 07:01