
Chemistry, 14.07.2019 01:00 morgan3346
Acetylene gas, c2h2, burns in oxygen to produce carbon dioxide and water. if 60.5 g co2 are produced when 22.6 g c2h2 react with sufficient oxygen, what is the percent yield for the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
Acetylene gas, c2h2, burns in oxygen to produce carbon dioxide and water. if 60.5 g co2 are produced...
Questions


Mathematics, 24.03.2020 01:30


Computers and Technology, 24.03.2020 01:30


Mathematics, 24.03.2020 01:30

Social Studies, 24.03.2020 01:30

Mathematics, 24.03.2020 01:30

Mathematics, 24.03.2020 01:30

Mathematics, 24.03.2020 01:30



English, 24.03.2020 01:30



Mathematics, 24.03.2020 01:32



Mathematics, 24.03.2020 01:32

Mathematics, 24.03.2020 01:32

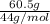
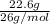
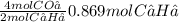
 x 100
x 100

