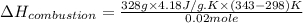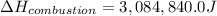Chemistry, 14.07.2019 02:00 autumperry7078
The energy from 0.02 moles of butane is used to heat 328 grams of water. the temperature of the water rose from 298 k to 343 k. (the specific heat capacity of water is 4.18 j/k g.) what is the enthalpy of combustion? a. -61.7 kj b. 1,578.01 j c. 3,084,840.0 j d. 23,513,336 j

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1


Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 24.06.2019 08:00
If an atom contains 11 protons and 12 neutrons, what is its mass number?
Answers: 1
You know the right answer?
The energy from 0.02 moles of butane is used to heat 328 grams of water. the temperature of the wate...
Questions


Mathematics, 22.11.2020 07:00




Mathematics, 22.11.2020 07:00

Chemistry, 22.11.2020 07:00



Chemistry, 22.11.2020 07:10

SAT, 22.11.2020 07:10

Chemistry, 22.11.2020 07:10


Mathematics, 22.11.2020 07:10

Chemistry, 22.11.2020 07:10

Mathematics, 22.11.2020 07:10

Chemistry, 22.11.2020 07:10


Social Studies, 22.11.2020 07:10

Mathematics, 22.11.2020 07:10

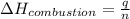
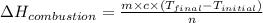
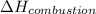 = enthalpy of combustion = ?
= enthalpy of combustion = ? = specific heat of water=
= specific heat of water= 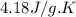
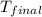 = final temperature = 343 K
= final temperature = 343 K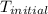 = initial temperature = 298 K
= initial temperature = 298 K