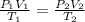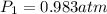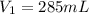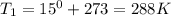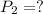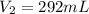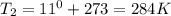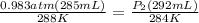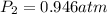Chemistry, 14.07.2019 02:30 ethanyayger
Astudent collects 285 ml of o2 gas at a temperature of 15°c and a pressure of 0.983 atm. the next day, the same sample occupies 292 ml at a temperature of 11°c. what is the new pressure of the gas? 0.946 atm 1.00 atm 0.704 atm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Astudent collects 285 ml of o2 gas at a temperature of 15°c and a pressure of 0.983 atm. the next da...
Questions


Mathematics, 05.09.2020 01:01

Mathematics, 05.09.2020 01:01

History, 05.09.2020 01:01

English, 05.09.2020 01:01

Biology, 05.09.2020 01:01

Mathematics, 05.09.2020 01:01

English, 05.09.2020 01:01

History, 05.09.2020 01:01



Mathematics, 05.09.2020 01:01



History, 05.09.2020 01:01

English, 05.09.2020 01:01

Mathematics, 05.09.2020 01:01