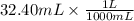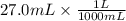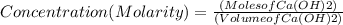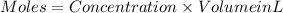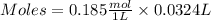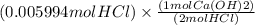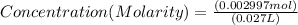Chemistry, 14.07.2019 02:30 fespinoza019
If 27.0 ml of ca(oh)2 with an unknown concentration is neutralized by 32.40 ml of 0.185 m hcl, what is the concentration of the ca(oh)2 solution? show all of the work needed to solve this problem. (2 points) ca(oh)2 + 2hcl yields 2h2 o + cacl2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1
You know the right answer?
If 27.0 ml of ca(oh)2 with an unknown concentration is neutralized by 32.40 ml of 0.185 m hcl, what...
Questions


Health, 14.07.2019 15:30

Mathematics, 14.07.2019 15:30

Chemistry, 14.07.2019 15:30





Geography, 14.07.2019 15:30


Chemistry, 14.07.2019 15:30





Physics, 14.07.2019 15:30



Mathematics, 14.07.2019 15:30