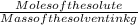Chemistry, 14.07.2019 04:00 alexcute965
Asolution is made by dissolving 21.5 grams of glucose (c6h12o6) in 255 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3
You know the right answer?
Asolution is made by dissolving 21.5 grams of glucose (c6h12o6) in 255 grams of water. what is the f...
Questions


Computers and Technology, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00

Social Studies, 07.07.2019 16:00





Mathematics, 07.07.2019 16:00





Biology, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00




Biology, 07.07.2019 16:00