
Chemistry, 14.07.2019 04:00 gilbert325
If 3.5 moles of nitrogen monoxide (no) react with 6.0 moles of oxygen gas (o2), how many moles of the product can be formed and how many moles of the excess reactant will be left over when the reaction is complete? show all of your work. unbalanced equation: no + o2 “yields”/ no2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1


Chemistry, 23.06.2019 02:00
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2
You know the right answer?
If 3.5 moles of nitrogen monoxide (no) react with 6.0 moles of oxygen gas (o2), how many moles of th...
Questions

Mathematics, 08.03.2021 19:50




Mathematics, 08.03.2021 19:50

Mathematics, 08.03.2021 19:50

Social Studies, 08.03.2021 19:50

Mathematics, 08.03.2021 19:50

History, 08.03.2021 19:50

Social Studies, 08.03.2021 19:50



History, 08.03.2021 19:50



English, 08.03.2021 19:50

History, 08.03.2021 19:50



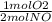 x 3.5 mol NO
x 3.5 mol NO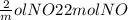 x 3.5 mol NO
x 3.5 mol NO

