
Chemistry, 14.07.2019 06:00 achoward08
In the hypothetical reaction below, substance a is consumed at a rate of 2.0 mol/l·s. if this reaction is at dynamic equilibrium, at what rate will substance b be consumed? 0.0 mol/l·s 1.0 mol/l·s 2.0 mol/l·s 4.0 mol/l·s

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
In the hypothetical reaction below, substance a is consumed at a rate of 2.0 mol/l·s. if this reacti...
Questions








History, 18.07.2020 01:01


Mathematics, 18.07.2020 01:01





Mathematics, 18.07.2020 01:01



History, 18.07.2020 01:01


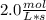
![R(a) = k * \frac{d[A]}{dt}](/tpl/images/0087/5945/2a16d.png)
![R (b)= k * \frac{d[B]}{dt}](/tpl/images/0087/5945/bbb74.png)



