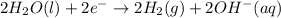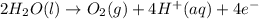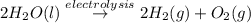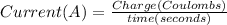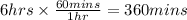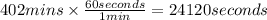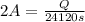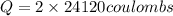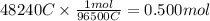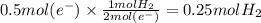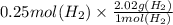Chemistry, 14.07.2019 10:00 nekathadon
In the electrolysis of water shown below, a current of 2 amps is applied to 180 ml of h2o(l) for 6 hours and 42 minutes. how many grams of h2(g) are formed? (faraday's constant = 96,500 c/mol)

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
If this equation was completed which statement would it best support
Answers: 1

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1
You know the right answer?
In the electrolysis of water shown below, a current of 2 amps is applied to 180 ml of h2o(l) for 6 h...
Questions

SAT, 26.09.2021 14:00


Mathematics, 26.09.2021 14:00


Social Studies, 26.09.2021 14:00



Mathematics, 26.09.2021 14:00

Physics, 26.09.2021 14:00

Physics, 26.09.2021 14:00

Chemistry, 26.09.2021 14:00

History, 26.09.2021 14:00

Computers and Technology, 26.09.2021 14:00


Mathematics, 26.09.2021 14:00



Mathematics, 26.09.2021 14:00

Mathematics, 26.09.2021 14:00

English, 26.09.2021 14:00